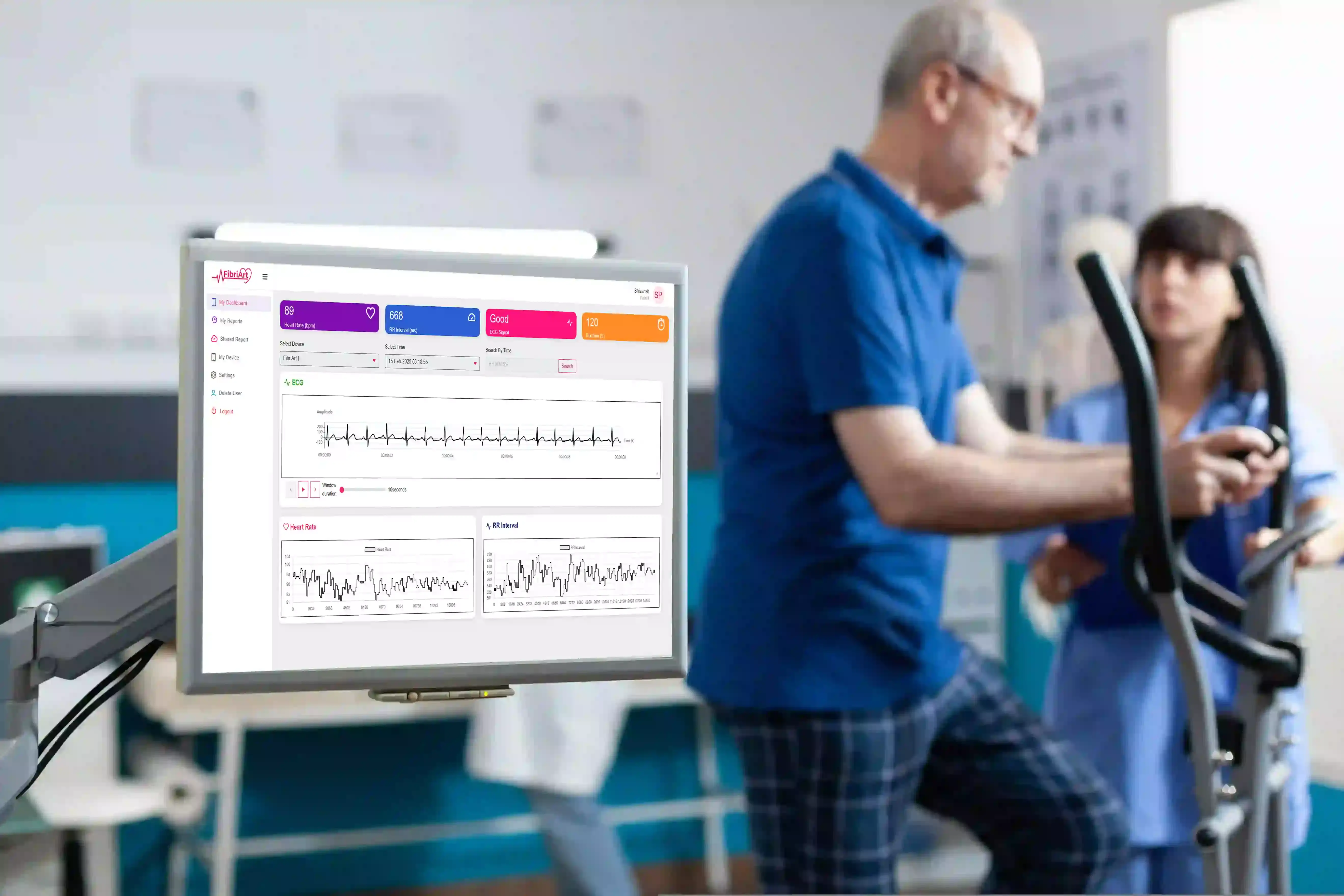
Wearable cardiac monitoring technologies have expanded the reach of cardiovascular diagnostics beyond the hospital. Cardiologists today are seeking devices that not only meet clinical-grade standards for accuracy but also offer patient-friendly design and streamlined integration into care workflows. This article explores what clinicians expect from wearable ECG devices and how emerging technologies such as those offered by FibriArt are aligning with these expectations.
1. Clinical-Grade Accuracy for Arrhythmia Detection
Diagnostic accuracy is the foremost concern for cardiologists evaluating wearable heart monitors. Devices must deliver high sensitivity and specificity for detecting atrial fibrillation (Afib), bradyarrhythmias, premature contractions, and more.
- Consumer wearables such as smartwatches report Afib detection sensitivities around 78?88%, which may be suitable for screening but fall short for clinical diagnosis.
- Clinical-grade wearable ECG patches generally report sensitivities of 93?97% and specificities of 95?98% for arrhythmia detection, according to peer-reviewed studies (MDPI, Healthcare Bulletin).
Clinicians prefer devices that record and analyze full PQRST waveforms, rather than RR interval variability alone. Full-wave data enables the identification of rhythm abnormalities with greater nuance and clinical utility.
2. Extended Monitoring to Capture Intermittent Events
Arrhythmias often occur unpredictably and may not manifest during short-term evaluations. Cardiologists, therefore, favor devices that support long-duration monitoring without sacrificing comfort or signal quality.
- Standard Holter monitors record for 24 to 48 hours, often missing transient arrhythmias.
- Newer wearable solutions, such as the FibriArt X, offer up to 28 days of continuous monitoring, improving the likelihood of detecting paroxysmal Afib and other episodic conditions.
Extended monitoring duration is particularly valuable for post-operative patients, those with cryptogenic stroke, or individuals under medication adjustment.
3. Patient Comfort and Compliance
Even the most accurate device is ineffective if patients cannot wear it consistently. Comfort and usability significantly influence data quality and compliance in remote cardiac monitoring.
Clinicians look for devices that are:
- Lightweight and skin-friendly ? Reducing irritation and improving wear time.
- Discreet and waterproof? Allowing patients to carry on normal activities, including bathing and exercise.
- Easy to maintain ? With battery life that supports uninterrupted multi-day use.
FibriArt?s wearable ECG patch addresses these needs through a textile-based conductive ink design, which eliminates metal electrodes and reduces motion artefacts. The patch is also water-resistant and designed for weekly replacement, promoting patient comfort over long-term use.
4. Remote Monitoring with Integrated Reporting
Cardiologists increasingly rely on remote cardiac monitoring to deliver care outside traditional clinical settings. This requires not only robust data capture but also efficient data transmission and analysis.
Essential features include:
- Automatic wireless uploadof ECG data to secure cloud platforms.
- Real-time event alerts for critical findings such as Afib or bradycardia.
- Clinician-facing dashboardsthat prioritize actionable events and minimize time spent reviewing non-clinical segments.
FibriArt?s physician dashboard enables cardiologists to quickly interpret rhythm data, download structured reports, and integrate findings into electronic health records (EHR). This helps streamline workflow, reduce manual review, and support faster decision-making.
5. Regulatory Alignment and Clinical Validation
Trust in wearable ECG devices depends on adherence to medical regulatory standards and independent clinical validation.
Key considerations include:
- FDA 510(k) clearanceandCE Marking, which validate safety and efficacy.
- Conformity withIEC 60601-2-47 andISO standards for cardiac monitoring.
- Compliance withHIPAAandGDPR to protect patient data privacy.
FibriArt is actively pursuing FDA clearance and already designs its products in alignment with international standards. The company also engages in ongoing clinical comparisons to benchmark its system against Holter monitors and other conventional ECG technologies.
How FibriArt Aligns with Cardiologists' Expectations
| Clinical Need | FibriArt Response |
|---|---|
| Accurate detection of arrhythmias | Full-waveform PQRST analysis; designed for clinical-grade monitoring. Independent validation pending |
| Extended wear with minimal discomfort | Up to 28-day monitoring; Daily reports, Ultra-low power, Water-resistant and sweat-resistant |
| Comfortable design for real use | Lightweight, Water-resistant, flexible adhesive, skin-friendly materials |
| Efficient data review and reporting | AI-supported arrhythmia classification, real-time alerts, structured reports |
| Regulatory compliance and safety | FDA 510(k) pathway, IEC 60601 standards, HIPAA/GDPR compliance |
Common Clinical Applications
Cardiologist-preferred wearable tech is increasingly integrated into the following care workflows:
-
Palpitation or syncope evaluation
Long-duration rhythm monitoring for identifying transient arrhythmias.
-
Afib screening in high-risk populations
Particularly in patients over age 65 or with hypertension or diabetes.
-
Post-ablation follow-up
Monitoring for arrhythmia recurrence during early recovery phases.
-
Medication management
Evaluating the efficacy and safety of antiarrhythmic drugs over time.
-
Cryptogenic stroke investigation
Identifying subclinical Afib as a potential cause.
FibriArt?s system supports each of these clinical scenarios with ease of use, extended wear, and reliable ECG capture.
Frequently Asked Questions (FAQs)
Wearable ECG monitors, when validated and correctly used, can offer diagnostic accuracy comparable to traditional Holter monitors. Devices like FibriArt, which provide full-waveform ECG and extended wear, achieve >90% sensitivity and specificity for arrhythmias such as Afib.Source: American Heart Association
Wearable ECG patches can detect common rhythm abnormalities such as Afib, atrial flutter, bradycardia, and ectopic beats. However, more complex conduction disorders or ischemic changes may require a multi-lead ECG. Source: Mayo Clinic
Yes, several devices are FDA-cleared or CE-marked. FibriArt is in the process of obtaining regulatory approvals and designs per IEC and ISO medical device standards. Source: FDA Medical Device Database
Depending on the device, wear duration can range from a few days to several weeks. FibriArt offers up to 28 days of monitoring through modular patches that are changed weekly for comfort and signal reliability. Source: NIH Wearable Cardiac Tech Overview
Data is securely transmitted to encrypted cloud servers. Clinicians receive automated reports highlighting relevant findings, and patients can be alerted to critical issues when needed. Source: World Health Organisation on Digital Health